FIRE: Is Your Project Ethical?
Ethical concerns in human subjects research center around participant welfare and informed consent. Key considerations include:
Informed Consent
Ensuring participants have a clear understanding of the study’s purpose, procedures, risks, and benefits before agreeing to participate. In some cases, if your participants are under age 18, they may need to get written permission from their parent or guardian to take part in your study.
Privacy and Confidentiality
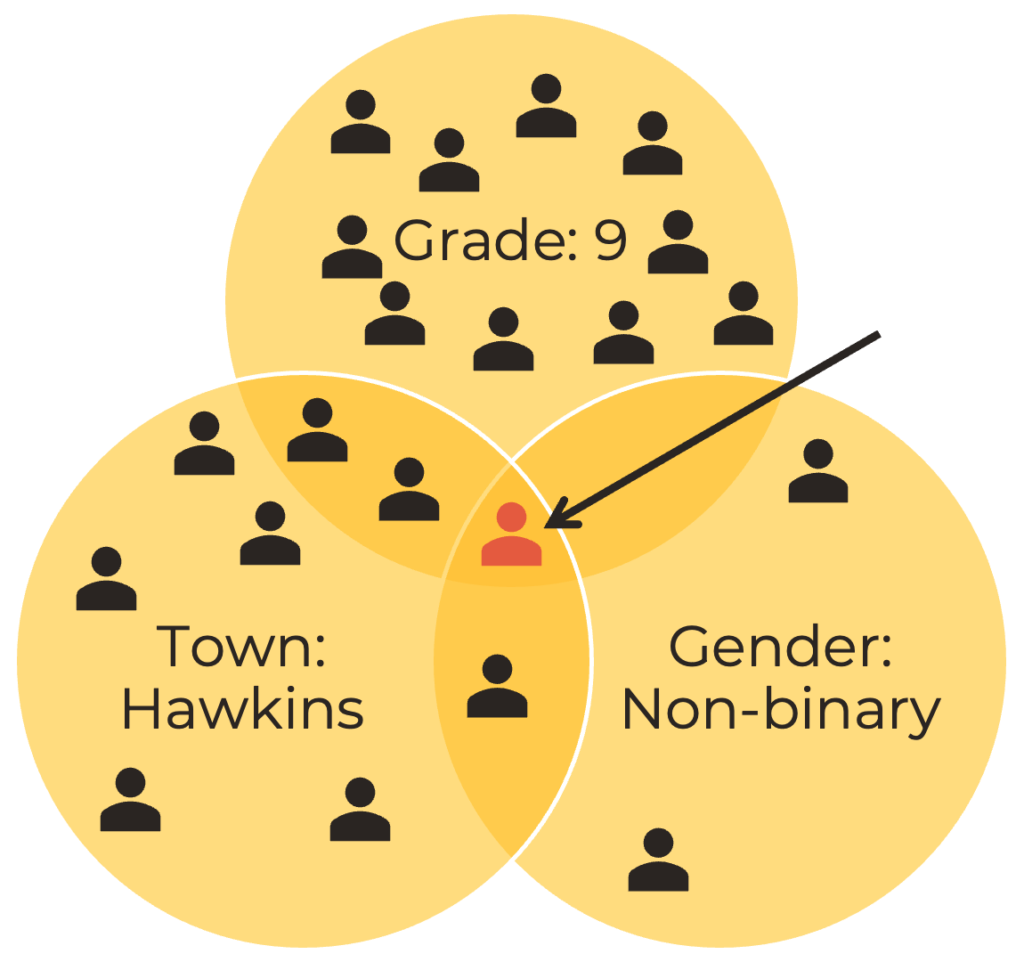
Safeguard participants’ personal information and ensure that their data remains confidential. Surveys need to be anonymous. Beyond asking a person’s name, you should avoid asking too many specific questions that could identify an individual. For example, if you ask the student’s grade, town, and gender, it could be possible to identify who that person is. It is important to only ask questions that are directly tied to your research question. If your question has nothing to do with gender identity, for instance, you should not ask that question of your participants. Learn more about creating surveys in our Survey Design video.
Minimizing Harm
You must design your experiment to minimize any potential physical, psychological, or social harm to participants during the research process. This includes asking questions about topics such as trauma, abuse, mental health, drug use, and criminal activity. The MSSF IRB will look for a clear plan to keep your participants safe in all regards.
Equitable Selection
Be sure that you are selecting your participants fairly. This is important to avoid bias in your data and hear from people with different viewpoints and life experiences.
Respect for Autonomy
You must never force someone to take part in your study, and those who do not want to take part should not face any negative consequences. Respect your participants’ privacy and the freedom to stop participating in the study at any time.
Benefit-Risk Assessment
Weigh the potential benefits of your study against any risks to your participants. While the IRB will assess your project for risk, you should do a thorough risk assessment of your own before you submit the project for approval.
Data Integrity and Analysis
You are responsible for being accurate and unbiased in your data collection, analysis, and how you share your findings. You also must protect your data so that it does not fall into the wrong hands. This is also why it is important to avoid asking too many identifying questions, as the information could be used to cause harm to your participants.
By addressing these ethical concerns, researchers uphold the principles of integrity, respect, and responsibility. This builds trust between researchers and participants while advancing knowledge for the greater good.
True Story: Henrietta Lacks

In 1951, without her knowledge or consent, doctors took cells from Henrietta Lacks, a young African American woman suffering from cervical cancer. These cells exhibited an unprecedented ability to replicate indefinitely, leading to significant advancements in medical research and the development of vaccines, treatments, and scientific discoveries. These “HeLa” cells are still being used today. The use of Lacks’ cells without her consent sparked ethical debates surrounding patient rights and informed consent. Her story has raised awareness about the ethical use of human tissue in research and led to discussions on the importance of informed consent and privacy (source).
Now it's time for a quiz!
Click on the quiz below to begin. You can take this quiz as many times as you need to to get a score of 80 or higher.
